Once you receive approval to advance your device to the clinical stage, you will have many new questions to address, such as:
- What are the right endpoints to collect and report to best support the objectives of my trial?
- How can I identify and qualify preferred sites to ensure enrollment and study compliance success?
- What is the best patient population and how can I motivate patients to participate in my trial?
Put your clinical strategy into action with support for pilot/feasibility, pivotal and post-market device trials. Your trial is seamlessly conducted - from study design consultation and protocol all the way through to the final analysis and summary report for regulatory submission.
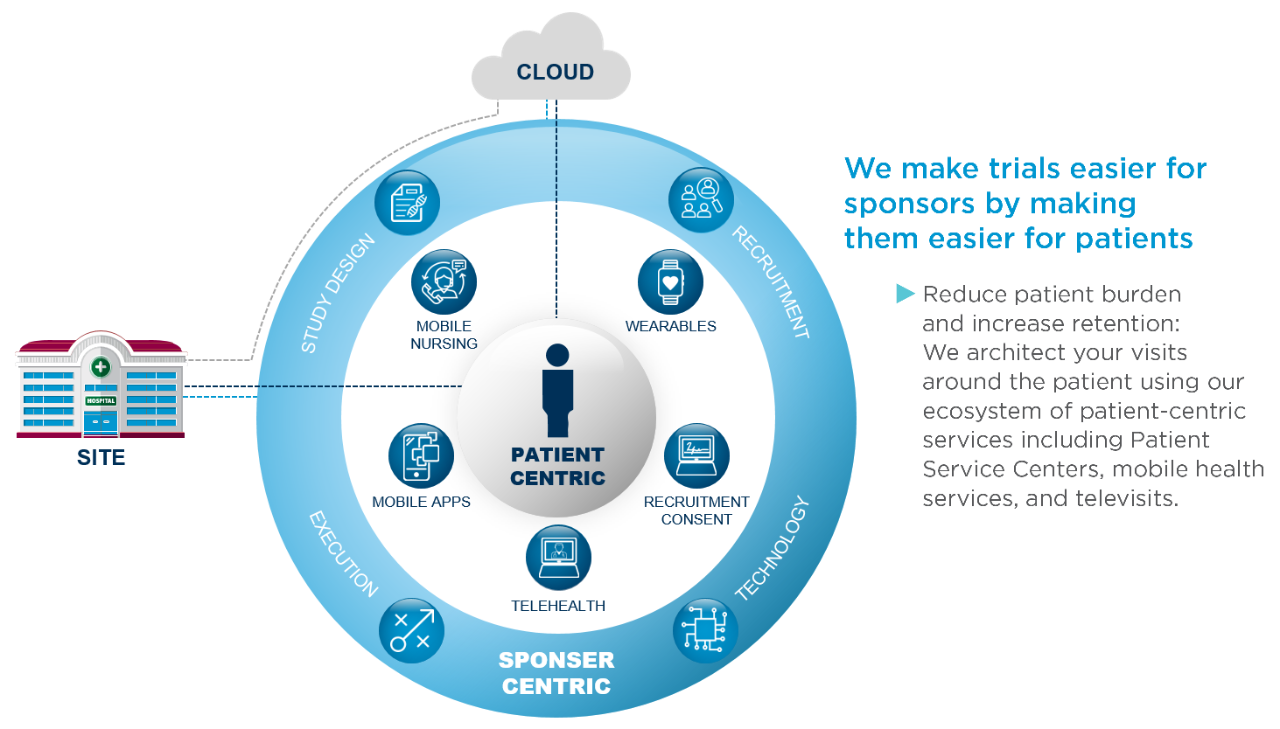
Advance smartly with a dedicated clinical team that specializes in device trials. Your study is driven with strong science and deep care area experience to deliver a patient-centric trial. Plus, unique data sources are leveraged to inform and accelerate the recruitment of high-performing investigators and patient populations.
With innovative approaches from traditional to decentralized (virtual) trials, rest assured you will experience an expertly executed clinical study tailored to your specific clinical areas.
Customized trials to fit your clinical discipline: